Most vaccines require a series of multiple shots before the recipient can be considered fully vaccinated. The most recent example of this is the COVID-19 vaccine, for which most people require multiple doses. Now, scientists are developing a one-jab, self-boosting vaccine that can deliver several vaccine doses over time.
Scientists from the Massachusetts Institute of Technology (MIT) say they developed microparticles that release payloads at different intervals, which could be used to create "self-boosting" vaccines.
In a new study published in the journal Science Advances, the researchers explained that using these microparticles that resemble tiny coffee cups sealed with a lid, they can design vaccines that need to be administered just once and would then "self-boost" at a stipulated time in the future. These particles would remain under the skin until the vaccine is released and then break down. According to the team, the jab could fight numerous illnesses, from measles to COVID.
Related: Is It Possible to Create a UNIVERSAL Vaccine?
They further state that this type of vaccine delivery can be handy in administering childhood vaccinations in those areas where people don't have easy access to medical care. It can be useful for delivering a variety of other therapies, including cancer drugs, hormone treatments, and other medications.
"This is a platform that can be broadly applicable to all types of vaccines, including recombinant protein-based vaccines, DNA-based vaccines, even RNA-based vaccines," says Ana Jaklenec, a research scientist at MIT's Koch Institute for Integrative Cancer Research. "Understanding the process of how the vaccines are released, which is what we described in this paper, has allowed us to work on formulations that address some of the instability that could be induced over time."
How does the multi-dose shot work?
The researchers explain that the microparticles are made from PLGA, a biocompatible polymer approved for use in such medical devices as implants, sutures, and prosthetic devices. The team created arrays of silicon molds to shape the PLGA "cups" and "lids." After forming the polymer cups, the researchers used a custom-built, automated dispensing system to fill each cup with a drug or vaccine. Once the cups were full, the lids were aligned and lowered onto each cup. Meanwhile, the system was heated slightly until the cup and lid fused together, allowing the drug to be sealed inside. This technique is called SEAL (StampEd Assembly of polymer Layers), and it can be used to produce particles of any shape or size.
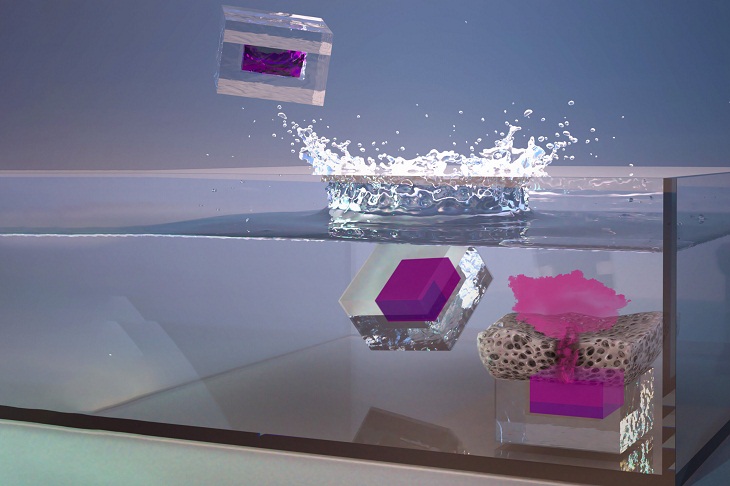
Image source: Second Bay Studios (Edited by MIT News)
The team’s analysis of the release mechanism revealed that the PLGA polymers that make up the particles are steadily cleaved by water. When enough of these polymers have broken down, the lid becomes porous. A short while later, the material breaks down, allowing the contents to spill out.
The researchers then analyzed how several different design parameters, including the size and shape of the particles and the composition of the polymers influence the timing of drug release.
The researchers were surprised to find that particle size and shape had a minimal effect on drug release kinetics. They said that if a particle has to be released after six months, a corresponding polymer can be used. If it has to be released after two days, another polymer can be used. “A broad range of applications can benefit from this observation,” they note.
Ironing out the issues
The researcher team is now working on ways to correct some technical problems they found in their approach. For example, they discovered that a change in the environment's pH affects the particles. When water breaks down the PLGA polymers, the byproducts, including lactic acid and glycolic acid, make the overall environment more acidic. This can damage the drugs carried within the particles (usually, proteins or nucleic acids). The team hopes that they will be able to counteract this increase in acidity and improve stability.

The team also developed a computational model to help with future particle design. This model can predict how a particular particle will degrade in the body, taking various parameters into account. The model could potentially guide the development of other similar microfabricated or 3D-printed particles and medical devices.
The research team has already used this approach to design a self-boosting polio vaccine, which is currently tested in animals. Usually, polio vaccines have to be administered as a series of two to four separate injections.
The team is confident that the core-shell particles can help create a safe, single-injection, self-boosting vaccine. A combination of vaccines with different release times can be produce by altering the composition of the microparticles. “Such a single injection approach has the potential to not only improve patient compliance, but also increase cellular and humoral immune responses to the vaccine," notes co-senior author Prof. Robert Langer.
Related: A Vaccine for Alzheimer's?
Last but definitely not least, the researchers have already shown that this type of drug delivery can be useful for treating diseases like cancer. In a 2020 Science Translational Medicine study, the researchers published a paper demonstrating how they can deliver drugs that trigger a pathway called STING. In several mouse models of cancer, it boosted immune responses. The particles delivered several doses of the drug over several months after being injected into tumors. According to the researchers, this inhibited tumor growth and reduced the spread of the disease.
Share this post with friends and family
 Go to BabaMail
Go to BabaMail