




Scientists Might Have Found Out a New Way to “Breathe”
In a stunning new experiment, German scientists harnessed the power of photosynthesis to teach an animal a new way to “breathe.”
 7:25
7:25
The Giant Ape You Never Heard About...
Gigantopithecus was the largest ape on our planet. But what exactly happened to this incredible creature?
 3:39
3:39
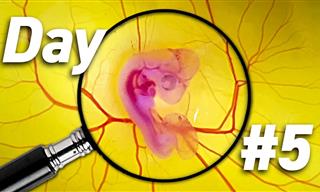
The Many Fascinating Stages in Chick Embryo Development
Ever wondered how a chicken exactly forms inside an egg? The whole process is actually fascinating...
 12:57
12:57
The Curious Science of Tricking Your Taste Buds
Did you know you could confuse your sense of taste? Find out how!

Doctors Make History With First Whole Eye Transplant
Surgeons in New York have performed the world’s first transplant of an entire human eye!
 8:52
8:52
Watch This Scientist Answer Some Fun Science Questions
In this video, scientist Bill Nye answers some interesting science questions.

Do You Have One Of These RARE Genetic Traits?
How unique are you? You might have several rare genetic traits that make you unlike most of the world's population!

17 Incredible Eye Facts You Have To See To Believe
They say the eyes are the window to the soul, and they also happen to be our windows to the world. Here are 17 insanely fascinating facts about eyes.
Can One Photo Really Tell How Good Your Eyesight Is?
Scientists say that this single photograph can tell you whether you have good vision or not. Who can you see in the image?

GUIDE: How Alcohol Affects Us, Drink by Drink...
In this article, we're going to discuss the exact amounts of alcohol in our blood and what they look like, including how each level affects us and our ability to function.

When Under a Microscope, Even Ordinary Things Seem Alien
Have you ever given any thought to what every day things look like under a microscope? Some of these images are truly beautiful - see for yourself in the images below.
 16:28
16:28
This Secret Invention Changed the Course of WW2
Let’s take a closer look at the proximity fuze, also called Vt Fuze, an invention that changed the course of the second World War.
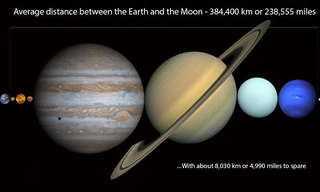
Amazing Science: THIS is the True Scale of Our Universe...
Get a sense of the true scale and shape of the universe we've discovered so far.
 12:35
12:35
The World's Most Dangerous Blood Type
Today we will discuss the most dangerous blood type to have, the one you cannot get an infusion for. Let's delve right in and learn about this rare blood type.

9 Great NASA Inventions That Took the World By Storm!
For around six decades, NASA have been inventing top-notch products. Here are nine great examples!

10 Tech Products You Must Double-Check Before Buying
Beware! These tech products are most commonly faked.
 5:32
5:32
The Science Behind Why Laughter is the Best Medicine
They say laughter is the best medicine. But why exactly do we laugh?

20 Fascinating Things That Can Be Found in the World
These pictures show us 20 fascinating and absolutely astounding things, from a single mountain with 1000 waterfalls to a giant ant!
Nobody Believed These 5 Scientists, But They Were Right
These 5 scientists were shamed and ousted, or rudely ignored, although years later, their "crazy" theories turned out to be true...

There are Some Great Secrets Found in Mathematics...
Numbers hold some great secrets - they even tell us about life!

Is Weekend Sleep as Good as Regular Sleep? Let's Check
If you thought sleeping in on the weekend was a bad habit, you're about to find out that it's quite the opposite.
 3:39
3:39
Magic is Only A Vibration Away With This Great Experiment
All it takes is some sand, a metal plate and carefully toned vibrations from a speaker to create stunning and complex patterns. This video shows you the amazing results!

2023 in Science: Biggest Discoveries You Missed!
Let’s take a look at the most amazing scientific discoveries that made news this year.
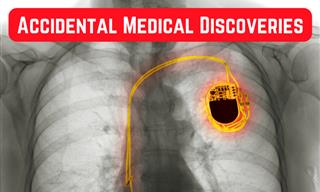
6 Fascinating Stories of Accidental Medical Discoveries
From penicillin to pacemakers, here are the medical breakthroughs that were discovered by accident.

10 New Things We've Learned About The Brain
We have learned some astonishing new things about the human brain in recent times.

I Never Knew These Stunning Facts About Our Universe
If you thought you understood the universe, you'll be completely floored by this insane facts.
 14:49
14:49
This Man Built an Automatic Train System for Trash Cans!
Wouldn’t it be cool if a robot picked up and moved our garbage out for pickup with the push of a button?

This Is Why You Should Choose Paper Towels Over Air Dryers
Jet air dryers seem to provide a rapid solution to drying our hands in public restrooms, but they're actually havens for bacteria. Take a look.
 5:08
5:08
Erecting a Behemoth: Installation of an Offshore Oilrig
How do they install an offshore oilrig? This video will show you, step by step, how it is done.

7 Weird Facts About the Human Voice That Will Surprise You
We bet you didn’t know these peculiar facts about the human voice.

The Difference Between Mined and Lab-Created Diamonds
If you're seriously considering buying a loved one a diamond, then we highly recommend that you read this informative guide first!

PayPal Versus Credit Cards: Here's the Lowdown!
Just how safe is PayPal? Should you have a PayPal account or should you pay for all online purchases using a credit card? All is revealed here!

5 Robotics Trends Anticipated in 2024
What does 2024 have in store for the field of robotics?
 13:38
13:38
24 Smart Storage Inventions You Didn't Know You Needed
These super useful inventions are designed to make you help space!
 6:31
6:31
The Psychology Behind Why Some People Are Habitually Late
Now we finally know why some people are habitually late.

7 Overlooked Scientists Who Should Be Household Names
Meet seven scientists who never got the credit they deserved.

6 Remarkable Yet Overlooked Minds That Shaped Our World
These underappreciated geniuses deserve more recognition.
 1:44
1:44
The Accidental Discovery of the World’s First Antibiotic
Not too long ago, it was fairly common to die of the simplest of wounds due to bacterial infection. Penicillin changed everything.
 23:26
23:26
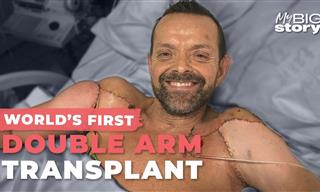
Incredible: This Man Got the First Double-Arm Transplant
Felix Gretarsson got burned in both arms and needed the new ones. For that purpose, he went through history's FIRST 2-arm transplant.
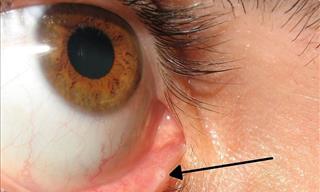
7 Little-Known Body Parts We Never Knew About
Here's a look at some of the weird and little-known body parts that you didn’t know you had.
 11:03
11:03
These New Technologies Will Change the World!
These groundbreaking tech innovations are going to change our world!
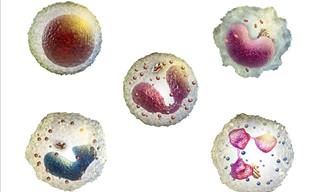
Let's Learn All About Your White Blood Cells
White blood cells (WBCs) are the part of your immune system which is responsible for fighting infection. Learn all about them here!
Six Inventions Da-Vinci Made Ahead of Their Time
He created and envisioned many inventions that were sometimes even centuries ahead of their time. Was there ever a genius as great as Da-Vinci?

Wow! Who Knew That Jupiter Was So Mesmerizing?
Take a look at Jupiter like you've never seen it before!
 21:07
21:07
15 Frightening Animals Early Humans Had to Live With
10 scary animals early cavemen in Africa had to deal with 200,000 years ago.

Study: New Link Found Between Parkinson's and the Gut
A recent study has identified gut microbes likely involved and linked them to decreased riboflavin (vitamin B2) and biotin (vitamin B7), suggesting a potential treatment
The Facts You Didn't Know About the Human Body...
Discover 120 fascinating and surprising facts about your body you may not have known. Going over most of the body parts, you will learn so much you never knew about the body we all use.


