
|
Science can truly be extraordinary, especially when you get an extreme chemical reaction. Here are 19 fabulous and seemingly inexplicable chemical reactions that you should definitely not try at home! |
| Prince Rupert's Drop Fracture |
 |
| The piece of glass known as Prince Rupert's Drop explodes from just a gentle tap, while the other end can withstand a hammer blow. The blub end can be hit repeatedly and not break, while the thin tail end makes the entire piece of glass shatter. This is because the glass is tightly compressed inside and explodes when there is strain of different temperatures. |
| White tin Crumbles into Grey Tin |
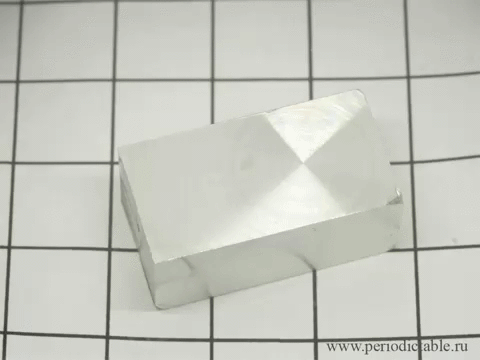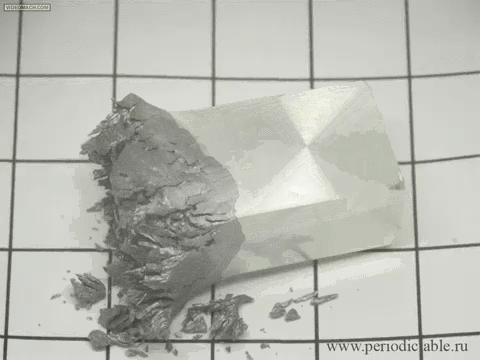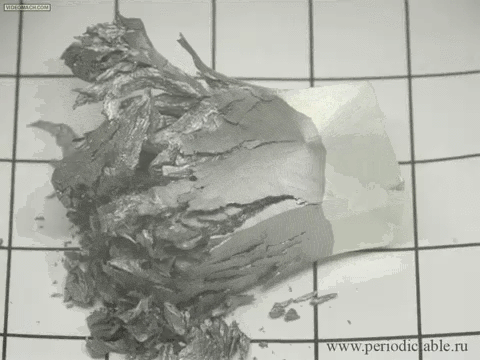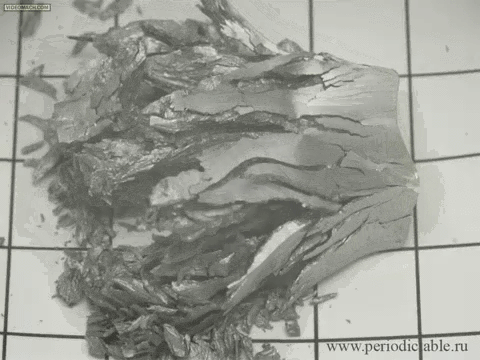 |
| Tin is a member of the carbon family, which means that it is highly affected by changes in temperature. When white tin is cooled to a temperature of less than 13 degrees Celsius (about 55 degrees Fahrenheit), it forms into grey tin. Although it seems to occur fast in this presentation, it actually occurs rather slowly, turning from its silvery, metallic material to a crumbling powder. |
| Elephant Toothpaste |
 |
| This experiment can easily be conducted at home and is a lot of fun! Elephant toothpaste is a foamy substance created by the rapid decomposition of hydrogen peroxide when it is mixed with liquid soap, or glycerin. The hydrogen peroxide breaks down into oxygen and water, but when the soap is added, it traps the oxygen in, creating the bubbles that turn into foam. |
| Mercury Reacts with Aluminum |
 |
| Mercury eats aluminum! Or so it appears from this demonstration. However the mercury does not really eat the aluminum, but rather penetrates the protective oxide layer of the aluminum making it rust much more rapidly. As the protective layer is constantly exposed to the air, the rusting process occurs rapidly, eating away at the metal. |
| Burning Ammonium Dichromate |
 |
| Ever wonder how they get those tabletop volcanoes at the science fair to explode? They do it by burning ammonium dichromate, a compound which is thermodynamically unstable, or reacts to heat. Once it is ignited, its decomposition reaction proceeds to near completion, which produces thick green powdered material, known as chromium oxide. |
| Hydrophobic Sand |
 |
| Also known as 'Magic Sand' or 'Mood Sand', this sand is coated with a hydrophobic compound, or molecules that repel masses of water. The presence of the hydrophobic compound causes the grains of sand to adhere to one another and form cylinders. As soon as the sand is removed from the water, it returns to its dry, free-flowing form. |
| Catalytic Decomposition of Hydrogen Peroxide |
 |
| Similar to the 'Elephant Toothpaste' experiment, when liquid soap or glycerin is added to hydrogen peroxide, this causes the oxygen to burst out and the water to turn into foam. However, this reaction usually takes some time and here, in order to make the effects more dramatic, a catalyst was added, or a substance that increases the rate of the chemical reaction. The result - a foam eruption! |
| Electrical Treeing |
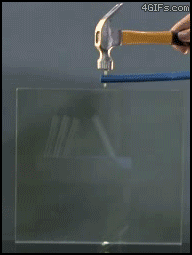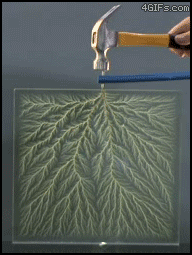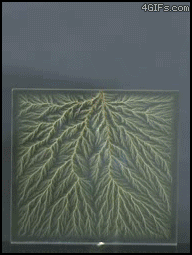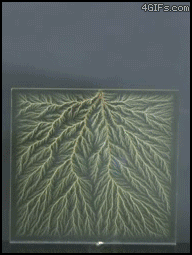 |
| In electrical engineering, treeing is an electrical pre-breakdown reaction when in a solid container. It is called 'treeing' because the electricity forms branches that resemble a tree. To put it a bit more simply, treeing occurs when electricity is trapped in a closed container and has nowhere to go, causing the circuits to break off and form 'branches'. |
| Burning Lithium |
 |
| The lithium in our batteries is a highly reactive chemical that we should be cautious of. When the chemical is burnt, it goes through spectacular changes, like turning the flame silver. In this experiment they used only a small amount of lithium because it is highly flammable and can be explosive when exposed to air and water. Don't try this at home because once you light the lithium on fire, it is very hard, if not impossible to extinguish without a dry powder fire extinguisher. |
| Burning Mercury(II) Thiocyanate |
 |
| This compound was once used in pyrotechnics, until the damaging effects of mercury were discovered. However, it still produces an awesome reaction called 'Pharaoh's serpent' when it is under heat. The heat causes a rapid exothermic reaction in the compound, which in turn produces a solid mass that looks like a coiling serpent. |
| Blood and Hydrogen Peroxide |
 |
| Ever put some hydrogen peroxide on a cut and watch it foam? This is because blood and cells contain an enzyme called catalase, which, when it comes into contact with hydrogen peroxide, turns the hydrogen peroxide into oxygen and water much like glycerin soap. If you put the hydrogen peroxide on a cut potato, the same thing will happen! |
| Sodium Acetate Crystallization |
 |
| Sodium acetate is commonly used in warming and heating pads and also in hot ice. The crystallization reaction occurs when the compound is cooled, which explains why you have to put your heating pad in the microwave once in a while to break up the crystals. In order for the crystals to melt, the compound needs to reach a temperature of 137.12 degrees Fahrenheit and 58.4 degrees Celsius. |
| The Belousov- Zhabotinsky Reaction |
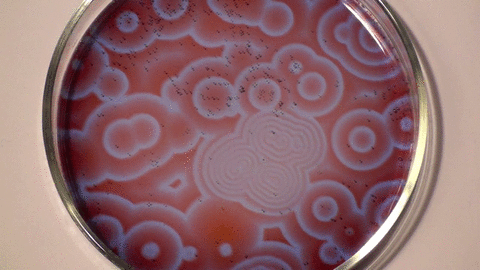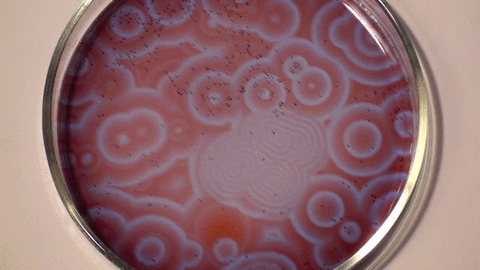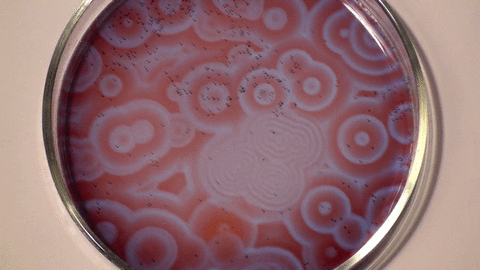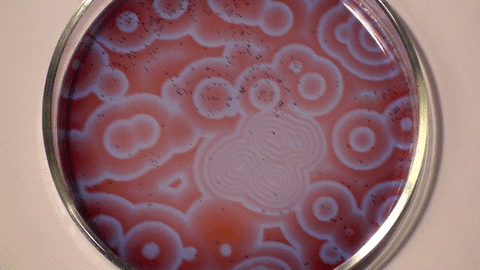 |
| The BZ reaction is a perfect example of non-equilibrium thermodynamics, or in plain speak, the reaction allows us to see the chemical processes unfolding before our eyes. The research showed that not all chemical reactions have involve a temperature element to occur and that some reactions occur indefinitely over a length of time. |
| Water Thread Experiment |
 |
| The water thread experiment occurs when two containers of deionized, or purified water, are placed on an insulator and one of the containers is given an electric charge, while the other has an electric charge. Once the electricity reaches a critical voltage, an unsupported water bridge forms between the two containers. The water bridge remains even when the containers are separated because the water strives to stabilize its electrical charge. |
| Particle Trails From Decay of Radon 220 |
 |
| When radon 220 is placed in a cloud chamber, or a particle detector used to study the effects of radiation, the environment inside the chamber is saturated with alcohol. When a charged alpha or beta particle travels through the alcohol gas, it leaves a trail of condensation behind it. In this case, radon 220 atoms decay into stable lead atoms and release two alpha particles, which produces the trails. |
| When Snake Venom Meets Blood |
 |
| Ever wonder why snake bites are so dangerous and deadly? When protein-rich snake venom is released into the human blood stream, it causes the blood cells to coagulate, or bind together and clot. The proteins in the snake's venom allow it to immobilize their prey and better digest their meal. However, because a snake cannot swallow a human whole (or at least hasn't until now), the effect of the venom in the blood stream is paralysis and blood coagulation as seen in the demonstration here. |
| Light Bulb Burns Out |
 |
| Light bulbs contain a filament, or that small piece sticking up in the middle of the bulb. In order to produce light, the filament is heated to white-hot temperatures by the electrical current that passes through it. However, when the electrical current is too strong, the filament gets so hot that it begins to melt and weaken. After the filament has been significantly weakened and worn out, it will suddenly explode when turning on the light, causing the light blub to burn out. |
| Aluminium and Iodine Reaction |
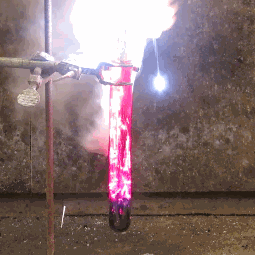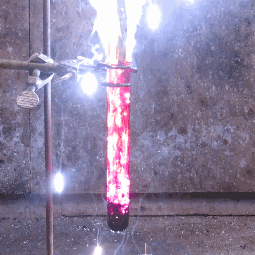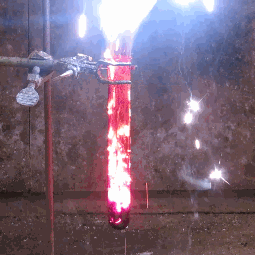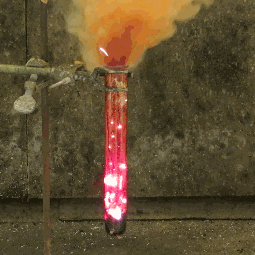 |
| When aluminium and iodine meet, both metal elements, and are combined with water, the result is clouds of purple iodine vapor. This is because together, the aluminium and the water break down the iodine into a deep violet gas. |
| Mixing Sugar and Sulfuric Acid |
 |
| Sugar is dehydrated when it meets sulfuric acid, meaning the water is removed from the sugar and a highly exothermic reaction of heat, steam and sulfur oxide fumes occurs. The white sugar becomes a balck carbonized tube that pushes itself out of the cup. This occurs because sugar is a carbohydrate, so when you remove all of the water from the molecule, you are left with carbon. |